Exploring Remote Clinical Research Careers: Tips for Success
Introduction
In the rapidly evolving field of clinical research, remote jobs offer professionals the unique opportunity to contribute to innovative health solutions from anywhere. Whether you’re interested in clinical data analysis, project management, or regulatory affairs, working remotely in clinical research is both rewarding and flexible. This guide will explore some of the exciting roles available, providing a glimpse into the diverse opportunities for building a meaningful career while enjoying the benefits of remote work.

Clinical Research Remote Job Opportunities
- Remote Clinical Data Manager
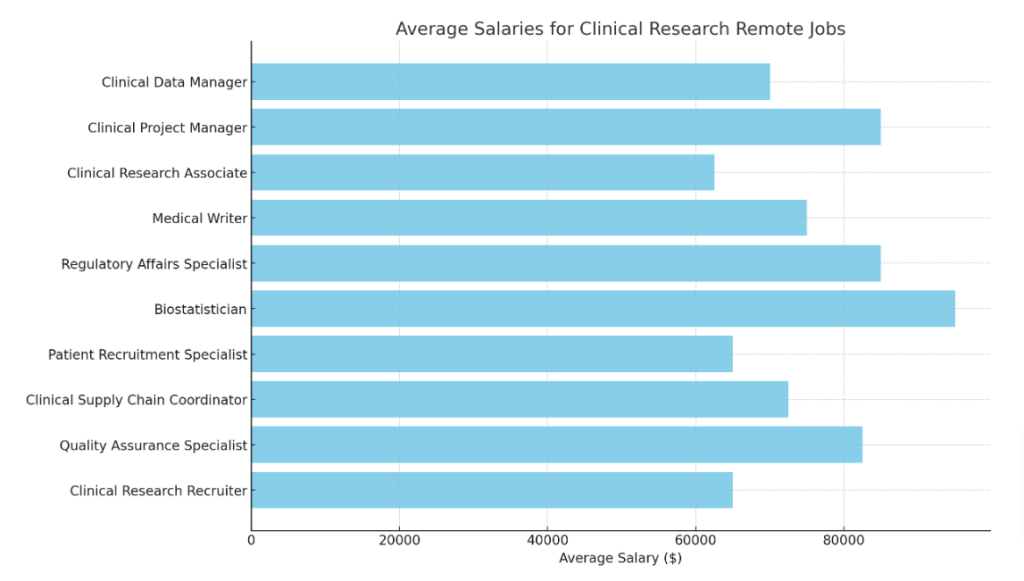
- Job Description: Oversee clinical trial data collection and ensure its quality and accuracy for compliance with protocols.
- Requirements: Experience with electronic data capture systems, analytical skills, bachelor’s degree in Life Sciences.
- Estimated Salary: $60,000-80,000/year
- Benefits: Flexible schedule, bonuses, and professional development.
- Virtual Clinical Project Manager
- Job Description: Manage clinical trial projects from planning to execution, including budgets, timelines, and team coordination.
- Requirements: Strong leadership, project management certification, relevant degree.
- Estimated Salary: $70,000-100,000/year
- Benefits: Leadership role, performance bonuses, supportive work environment.
- Telecommute Clinical Research Associate
- Job Description: Monitor clinical trial sites, ensure regulatory compliance, and liaise with research teams.
- Requirements: Knowledge of GCP guidelines, attention to detail, bachelor’s degree.
- Estimated Salary: $50,000-75,000/year
- Benefits: Travel opportunities, continuous training, supportive team.
- Remote Medical Writer
- Job Description: Develop clinical study reports, protocols, and other regulatory documents for clinical trials.
- Requirements: Exceptional writing skills, understanding of clinical research terminology, relevant degree.
- Estimated Salary: $60,000-90,000/year
- Benefits: Flexible work hours, creative freedom, health benefits.
- Regulatory Affairs Specialist (Remote)
- Job Description: Ensure clinical trial activities meet regulatory standards, prepare submissions for ethics committees.
- Requirements: Regulatory experience, bachelor’s degree in Life Sciences, strong communication skills.
- Estimated Salary: $70,000-100,000/year
- Benefits: Competitive salary, professional growth, work-life balance.
- Remote Biostatistician
- Job Description: Analyze clinical trial data to generate reports and statistical models that guide study outcomes.
- Requirements: Statistical analysis skills, familiarity with programming languages, master’s degree in Biostatistics.
- Estimated Salary: $80,000-110,000/year
- Benefits: Work on high-impact projects, supportive team culture, bonuses.
- Virtual Patient Recruitment Specialist
- Job Description: Design and execute patient recruitment strategies to ensure clinical trials reach their enrollment targets.
- Requirements: Marketing skills, experience in patient engagement, bachelor’s degree.
- Estimated Salary: $55,000-75,000/year
- Benefits: Travel opportunities, bonuses, work from anywhere.
- Remote Clinical Supply Chain Coordinator
- Job Description: Manage supply logistics, ensuring clinical trial sites receive the necessary materials and equipment.
- Requirements: Supply chain management experience, organizational skills, relevant degree.
- Estimated Salary: $60,000-85,000/year
- Benefits: Stable income, comprehensive benefits, leadership opportunities.
- Virtual Quality Assurance Specialist
- Job Description: Conduct audits, identify issues in clinical trials, and recommend corrective measures to maintain quality.
- Requirements: Quality assurance experience, attention to detail, bachelor’s degree.
- Estimated Salary: $70,000-95,000/year
- Benefits: Performance bonuses, flexible hours, supportive management.
- Remote Clinical Research Recruiter
- Job Description: Source, screen, and hire clinical research professionals to ensure trials are staffed with top talent.
- Requirements: Recruiting experience, excellent communication skills, bachelor’s degree in HR or Life Sciences.
- Estimated Salary: $50,000-80,000/year
- Benefits: Professional networking, work from home, comprehensive training.
To apply for any of these jobs or similar positions, click here.
What People Say
“Working as a virtual clinical project manager is perfect for me. The company values flexibility, and my team is supportive, even in a virtual environment. Sometimes, communicating across time zones can be tricky, but the sense of achievement after each successful trial makes it worthwhile.”
Valentine Ortiz, Duluth, Minnesota
“Being a remote medical writer has allowed me to use my skills in a rewarding way. The creative freedom is great, and my employer offers competitive pay and health benefits. I miss face-to-face meetings, but video calls keep me connected with my team.”
Chantelle Pierre, Middletown, Delaware
“As a remote clinical supply chain coordinator, I’ve been able to contribute significantly to the team’s efficiency while working from home. The pay is fair, and I appreciate the stability, although it’s sometimes challenging to keep up with changing regulations.”
Franklin Keene, Yukon, Oklahoma
“Telecommuting as a clinical research associate gives me the chance to travel while ensuring patient safety. Management is responsive and provides comprehensive training. The workload can be heavy at times, but the job is fulfilling.”
Liberty Sanchez, Lebanon, Oregon
“Managing quality assurance remotely has its challenges, but the company’s commitment to work-life balance makes it worth it. My role is essential, and I feel empowered by management to make decisions. The flexible hours help me juggle family life too.”
Evangeline Yeo, Penrith, Australia
More Remote Jobs
- Remote Healthcare Consultant
- Job Description: Advise healthcare organizations on improving patient outcomes, compliance, and operational efficiency.
- Requirements: Consulting experience, strong communication skills, relevant degree.
- Estimated Salary: $60,000-90,000/year
- Benefits: Professional development, leadership opportunities, performance bonuses.
- Virtual Clinical Site Manager
- Job Description: Oversee clinical trial operations at multiple research sites to ensure compliance with study protocols.
- Requirements: Management skills, clinical research knowledge, bachelor’s degree.
- Estimated Salary: $65,000-95,000/year
- Benefits: Supportive management, travel opportunities, comprehensive training.
- Remote Pharmacovigilance Specialist
- Job Description: Monitor and report adverse effects of clinical trial drugs, ensuring patient safety and regulatory compliance.
- Requirements: Pharmacovigilance experience, attention to detail, bachelor’s degree in Pharmacy or Life Sciences.
- Estimated Salary: $70,000-100,000/year
- Benefits: Work on cutting-edge projects, competitive salary, supportive team.
- Telehealth Coordinator
- Job Description: Support the delivery of telehealth services by managing schedules, patient data, and technical issues.
- Requirements: Familiarity with telehealth platforms, organizational skills, bachelor’s degree.
- Estimated Salary: $50,000-75,000/year
- Benefits: Comprehensive benefits, flexible hours, professional growth.
- Remote Medical Device Regulatory Specialist
- Job Description: Guide medical device manufacturers through regulatory approvals and ensure compliance with global standards.
- Requirements: Regulatory experience, attention to detail, relevant degree.
- Estimated Salary: $70,000-100,000/year
- Benefits: Bonuses, international opportunities, flexible schedule.
- Virtual Epidemiologist
- Job Description: Analyze data on disease outbreaks, develop strategies to reduce infection rates, and provide guidance to clinical teams.
- Requirements: Epidemiology expertise, analytical skills, master’s degree.
- Estimated Salary: $80,000-120,000/year
- Benefits: Work from anywhere, high-impact projects, continuous training.
- Remote Patient Safety Specialist
- Job Description: Monitor patient safety data, identify potential risks, and recommend improvements for clinical trial protocols.
- Requirements: Risk assessment experience, knowledge of GCP, bachelor’s degree.
- Estimated Salary: $60,000-90,000/year
- Benefits: Stable income, bonuses, supportive management.
- Telecommute Clinical Trials Finance Analyst
- Job Description: Manage budgets for clinical trials, ensure cost-effectiveness, and provide financial insights to project teams.
- Requirements: Financial analysis skills, relevant degree, attention to detail.
- Estimated Salary: $65,000-90,000/year
- Benefits: Comprehensive training, work from home, career growth.
- Remote Clinical Training Specialist
- Job Description: Develop and deliver training programs to enhance the skills of clinical research professionals globally.
- Requirements: Teaching experience, strong presentation skills, bachelor’s degree.
- Estimated Salary: $55,000-80,000/year
- Benefits: Creative role, international exposure, comprehensive benefits.
- Virtual Clinical Laboratory Specialist
- Job Description: Assist in managing clinical laboratory operations remotely, ensuring adherence to quality standards.
- Requirements: Laboratory management experience, relevant degree, attention to detail.
- Estimated Salary: $60,000-85,000/year
- Benefits: Comprehensive benefits, work-life balance, supportive culture.
To apply for any of these jobs or similar positions, click here.

Conclusion
In the world of clinical research, the abundance of rewarding remote job opportunities makes it an exciting field to pursue from anywhere. Whether you’re interested in managing clinical projects, ensuring patient safety, or navigating regulatory pathways, there’s a role that’s perfect for your skills and interests. By joining our email list, you’ll receive curated job listings, insightful career advice, and the latest industry trends directly to your inbox. Stay connected, stay informed, and explore the path to your next fulfilling career step. Sign up today!

